
Hair-thin muscles embedded in the skin of their wings allow bats like this Jamaican fruit bat to change the stiffness and curvature of their wings at different points of the wing stroke. That observation could help improve design of mechanical flight surfaces.
Credit: Swartz-Breuer lab/Brown University
Bats employ a network of nearly hair-thin muscles embedded in the membrane of their inherently floppy wing skin to adjust the wings' stiffness and curvature while they fly, Brown University researchers report. Birds and insects have stiff wings, but the new evidence suggests bats have evolved this muscular means of preserving or adjusting wing shape.
"Aerodynamic performance depends upon wing shape," said Brown biology graduate student Jorn Cheney, lead author of the newly published paper in Bioinspiration and Biomimetics. "The shape of a membrane wing might initially begin flat but as soon as it starts producing lift it's not going to remain flat because it has to deform in response to that aerodynamic load.
"The shape it adopts could be a terrible one – it could make the animal crash – or it could be beneficial," Cheney said. "But they are not locked into that shape. Because bats have these muscles in their wings, and also bones that can control the general shape as well, they can adopt any number of profiles."
Membrane muscle measurements
Cheney wasn't sure what to make of the tiny muscles, called plagiopatagiales, heading into the experiments reported in the paper. They have been known for more than a century but their function has never been demonstrated.
When Cheney considered the muscle function, he estimated that each individual muscle would be too weak to reshape the wing. That led him to form two competing hypotheses: either that the muscles would activate together to enhance force or that these oddly shaped, weak muscles might exist solely as sensors of stretch.
Only experiments could settle the question, so Cheney attached electrode sensors to a few muscles on the wings of a few Jamaican fruit bats and filmed them as they flew in the lab's wind tunnel.
Three key findings emerged from the data. They all point to the plagiopatagiales modulating skin stiffness.
One result was that the muscle activation and relaxation follows a distinct pattern during flight: They tense on the downstroke and relax on the upstroke.
"This is the first study showing that bats turn these muscles on and off during a typical wingbeat cycle," said co-author Sharon Swartz, professor of biology at Brown.
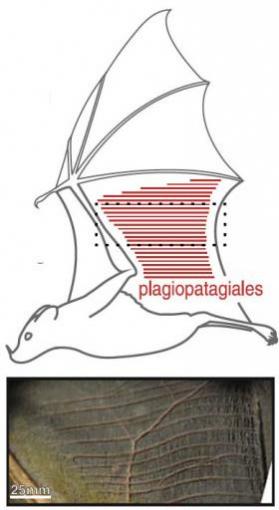 Wing surface changes during flight: Area of detail shows plagiopatagiales, tiny muscles that work together to stiffen or reshape an area of the bat's wing during the wingbeat cycle.Another finding was that the muscles don't act individually. Instead they exert their force in synchrony, providing enough collective strength to stiffen the wing.
Wing surface changes during flight: Area of detail shows plagiopatagiales, tiny muscles that work together to stiffen or reshape an area of the bat's wing during the wingbeat cycle.Another finding was that the muscles don't act individually. Instead they exert their force in synchrony, providing enough collective strength to stiffen the wing.
Finally, Cheney found, the muscles appeared to activate with different timing at different flight speeds. As the bats flew faster, they tensed the muscles sooner in the upstroke-downstroke cycle.
In other words, the data suggested that the muscles do not behave passively but actively and collectively in keeping with conditions of flight.
None of the data, however, preclude the muscles from serving a sensory function as well.
Technological insight, too
Cheney's findings fit into a larger program of research at Brown between the labs of biologist Swartz and co-author Kenneth Breuer, professor of engineering, in which, as Breuer puts it, they are "using biology to inspire engineering and using engineering to inspire biology."
In parallel with studies of real bats, the team has also built a robotic bat wing that incorporates their biological observations. Then they use the wing to generate data from experiments that they could never do with living creatures, such as precisely varying kinematic parameters like wingbeat frequency and amplitude, or the degree of wing folding during flapping.
In a separate paper in the same edition of Bioinspiration and Biomimetics, Swartz, Breuer, and former student Joe Bahlman report on how energy costs and aerodynamic forces changed as they varied several kinematic parameters in the robotic bat wing. They found that to generate a given force, such as lift, each of several parameters requires about the same amount of energy, but that the timing and extent of wing folding varies the ratio between lift and power significantly.
The group is now improving the robotic wing by integrating the new findings about how plagiopatagiales impact wing stiffness and shape.
"When one tries to build an engineered flying vehicle, you want to have control over its aerodynamic properties," Breuer said. "This is one more knob that we have to turn now. To be able to use these membrane wings and be able to control the properties in the way that we suspect bats do, using these muscles, is a great opportunity for biomimetic systems."
The group has a paper in press showing that controlling membrane tension in this way controls aerodynamic properties.
In addition to Cheney, Breuer, and Swartz, the paper's other authors are Erika Giblin, Nicolai Konow, and Thomas Roberts at Brown and Kevin Middleton at the University of Missouri.
The Air Force Office of Scientific Research (grants F49620-01-1-0335 and FA9550-12-1-0301) and the National Science Foundation (grant IOS1145549) funded the research.
- by David Orenstein