 Nurturing stem cells atop a bed of mouse cells works well, but is a non-starter for transplants to patients – Brown University scientists are developing a synthetic bed instead.
Nurturing stem cells atop a bed of mouse cells works well, but is a non-starter for transplants to patients – Brown University scientists are developing a synthetic bed instead.The most productive way scientists have devised to nurture colonies of human embryonic stem cells is to do so atop a bed of mouse cells. That may be fine for lab research, but it poses an unacceptable contamination risk for stem cells intended for transplant into human patients. In a new study, Brown University bioengineers have developed a synthetic bed that works about as well as the mouse cells, called fibroblasts, without any possibility of contamination.
"The gold standard for making the best stem cells would be starting with embryonic stem cells and growing them on a mouse embryonic fibroblast layer," said Diane Hoffman-Kim, associate professor of medical science and of engineering. "If we could understand the elements of that gold standard, then we could try to make an off-the-shelf product."
That potential product would be one that could advance stem cell therapies by taking mice out of the picture, said Hoffman-Kim, co-corresponding author with Eric Darling, also an associate professor of medical science and of engineering at Brown.
The researchers, led by Hoffman-Kim's former doctoral student Cristina Lopez-Fagundo, describe their advance in the journal Acta Biomaterialia. It turns out the key elements of the mouse fibroblasts that they learned to mimic were the stiffness of the cells and the bumpiness of the bed they formed.
By spreading a specially made material over the fibroblasts, the scientists created a rubbery mold. When they then used that to create beds with similar properties to a real one, they saw that that they could nurture and sustain comparable – though not identical – colonies of embryonic mouse stem cells on them.
"This is not the gold standard, and we're not saying it is," Lopez-Fagundo said, "But it definitely makes up for the disadvantages of culturing with mouse embryonic fibroblasts."
Measuring the mimic
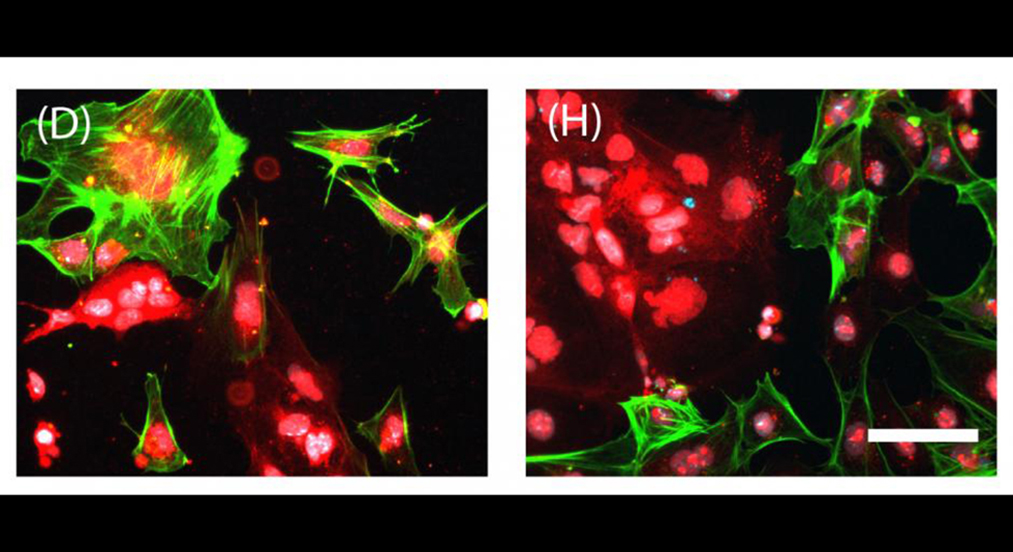
Experiments showed that the stem cell colonies nurtured on the synthetic beds were somewhat smaller in number at first but sometimes larger in area. By three weeks of culturing, both colony number and area were similar between the two. A third bed included as an experimental control, a surface with the optimal stiffness but no bumpiness, utterly failed to nurture the cells.
The next set of experiments tested whether the mouse embryonic stem cell colonies reared on synthetic or gold-standard fibroblast beds would mature comparably well. The team was able to show that they could induce stem cells from either bed to differentiate into each of the three "germ layers," which are precursors to tissue: the endoderm, which becomes internal body structures such as the gastrointestinal tract; the mesoderm, which becomes many organ systems, including the heart; and the ectoderm, which becomes the nervous system.
More to come
Hoffman-Kim and Lopez-Fagundo acknowledge they have more work to do before their advance can become useful for increasing stem cell production for clinical use. They've begun to test the synthetic bed with non-embryonic human stem cells that have similar potential to become different tissues as embryonic ones. Demonstrating the technology with human cells is a crucial step.
They also want to answer a more fundamental question: What is it about the stiffness of fibroblasts and the bumpiness of the layer they form that is so important in nurturing stem cell colonies? To learn that would be to reveal at least one of the many unknowns of stem cell biology.
"Right now in the field we are still at a frontier in terms of culturing and using stem cells," Hoffman-Kim said. "It's not fully resolved yet."
In addition to Hoffman-Kim, Lopez- Fagundo and Darling, the paper's other authors are senior research assistant Liane Livi and former master's student Talisha Ramchal.
The National Institutes of Health and the National Science Foundation funded the research.
by David Orenstein